
It took 14 days for my platelets to plummet back to a level low enough where I was beginning to bleed again. I had my first IVIg infusion at the beginning of the month, starting off at 20,000 platelets. My vein blew during the CBC beforehand. My insurance played games with approval, causing a four hour delay that ultimately rushed my treatment. 48 hours later, I was suffering with the classic symptoms of aseptic meningitis (don’t worry, an adverse event report has already been filed and I am okay).
My platelets reached 98,000 three days after the infusion, then 78,000 and 14 days later, back down to 21,000. Given all I went through, this was a disappointment. However, notably absent is my Sjögren’s related pain, so I won’t say this is a total loss. I declined doing one more infusion because I don’t believe a 14 day window is worth it. Ironically, Dr. Ahn predicted back in 2014 that an IVIg infusion would last no more than two weeks, again showing how absolutely brilliant he is!
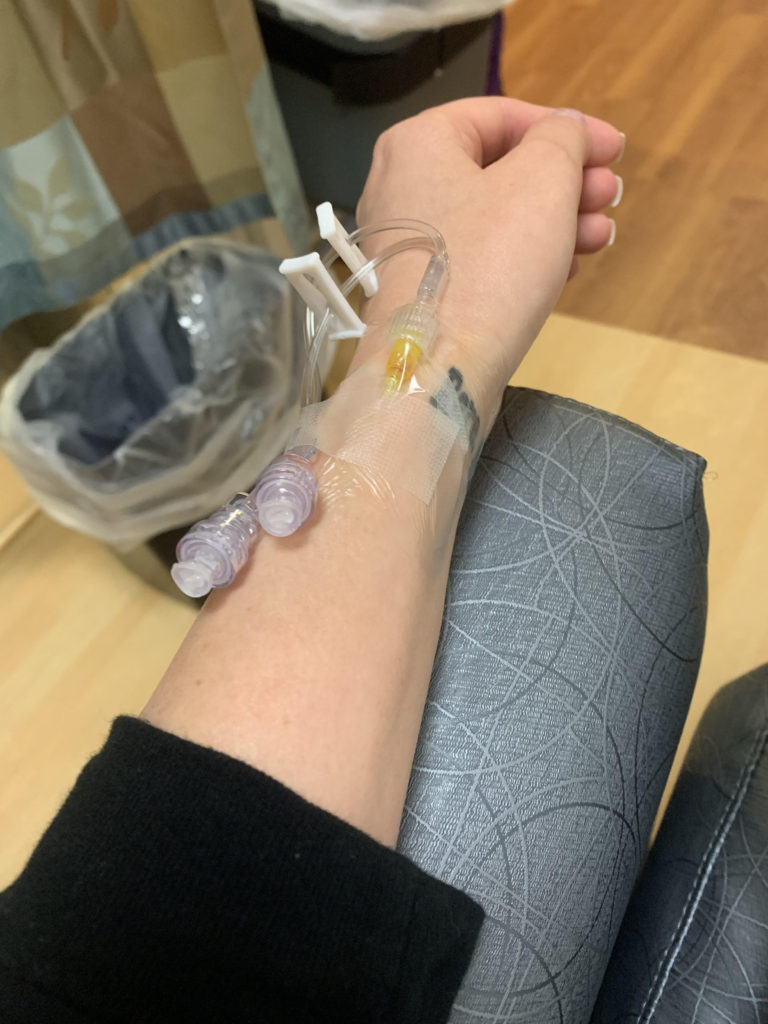
There are a few questions surrounding IVIg and how it will impact ITP patients with B-cell led destruction of platelets. I wonder if the prevalence of other autoimmune activity shortened the effectiveness of the infusion, or perhaps if I did two back to back, would it have lasted longer? I don’t feel the need to put myself in a lab rat position for this, simply because the scientific evidence is not compelling enough for a second look right now. I have my eyes set on the long term goal.
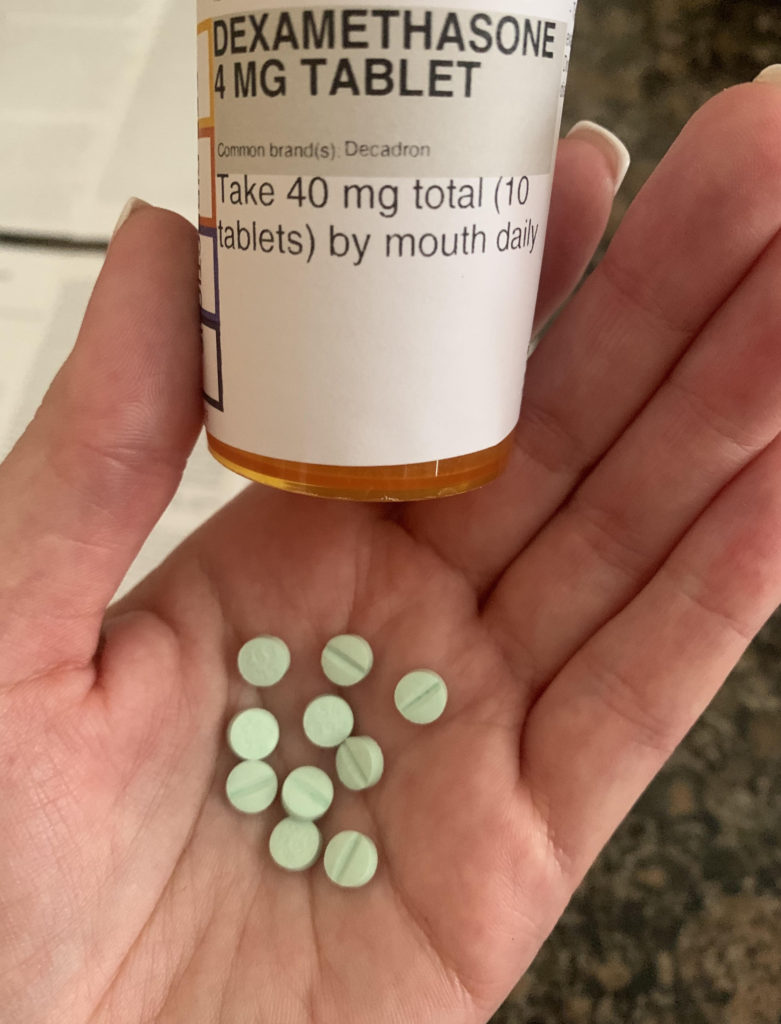
I ended up doing a Dex pulse for four days, and today is my last dose of 10 pills. This is not as bad as Prednisone in that it is quick, but it still comes with major side effects. Heartburn, insomnia, irritability, sweating, etc. Pretty much the same things I experienced before. Tomorrow morning I will have my platelets checked again, and I am curious to see how much they popped up.
Which brings me to the big news: we are trying Rituxan again. I have been waiting to hear those words since March, and I have dealt with a lot of miserable experiences to get here. This time is much different, I know what to expect, but the stakes are higher. I want to see Rituxan become an on-label treatment for patients with Chronic Immune Thrombocytopenia that have B-cell led destruction of platelets. That means it is not for people with T-cell issues, platelet production issues (who respond well to N-Plate and/or Promacta), and those on Tavalisse.
I launched the ITP Patient Driven Research Initiative last month to tackle this issue, and ultimately work toward better targeted therapies for patients with my rare disease. The research and drug development currently underway is a start, but we can do better. A solid data platform led by patients inputting their results based on a variety of treatments over extended periods of time will help us develop data sets needed for better treatment protocols. From there, we can acknowledge the clinical diversity of ITP and get pharmaceutical companies on board to run better clinical trials.
That’s the update for now. I have an early day at the hospital tomorrow with bloodwork and planning. I’m hoping to start infusing ASAP, especially since I have been through the platelet olympics these past few months.
