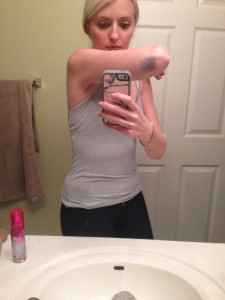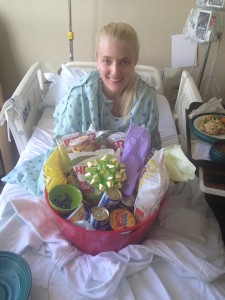
Allow me to introduce myself. My name is Candace Lerman and I’m a 28 year old rare disease patient. I lived 27 1/2 years as a happy and healthy person with a successful career, hobbies, lots of friends, the American dream. Then on May 1st, 2014 everything was taken from me. I was diagnosed with a rare blood disorder called Immune Thrombocytopenia (ITP). I had my whole life turned upside down because of it.
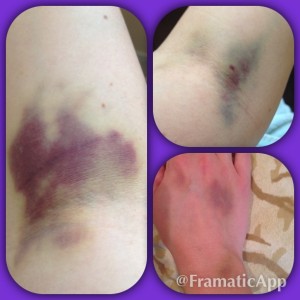
My disease can be painful and debilitating. I lived in a bubble while the best doctors in the world at the University of Miami tried to bring my disease under control. After seeing that steroids wouldn’t work and were destroying my body, my doctor recommended something that seemed impossible. He told me about Rituxan, a drug that treats Non-Hodgkins Lymphoma patients, and he thought it could help me control ITP. I was shocked, I was beyond scared and I was worried about what “chemo” would do to my already frail body.
I had to pray a lot, research a lot, think A LOT. I took a leap of faith and decided to go through with the treatment. I started this blog and documented everything, I wanted people to see what my experience was like using an FDA approved drug, off label. When I was declared in remission, I was elated. Finally I could begin to rebuild my new normal as a rare disease patient. I no longer had to live within the confines of ITP. I no longer had to fear dying too soon. The treatments were easy to deal with, and after four weeks I was an entirely new person. I finally established my new identity as a Rare Disease Warrior.

I traveled to DC for Rare Disease Week at the end of February and had the chance to meet with one of the sponsors of the OPEN Act, Congressman Bilirakis. I was moved to tears after telling him my story and our discussion of how repurposing FDA approved drugs for rare disease patients could save lives. I finally felt that all of the pain, the suffering, fear, heartache and sacrifices I made were going to contribute to a great cause. I don’t want anyone else with ITP or any other rare disease to experience the hopelessness I felt.
Shortly after my 28th birthday, before I ever knew I had another option, I contemplated killing myself. I’m not saying this to be dramatic, but I felt that there was no way out of the hell I was stuck in. I felt like I wouldn’t be able to live a normal life with the treatment options I had available. I wanted to go to law school, hang out with friends, spend time with my boyfriend, travel and experience all the wonderful things being 28 has to offer. I didn’t think I would have a chance to do any of it. I thought I would spend the rest of my life in and out of the hospital and my doctor’s office.
Rituxan has allowed me to be myself and I don’t see why other patients like me shouldn’t have the same opportunity I had. I am starting to worry about continued access to Rituxan once my remission ends. I have to change insurance plans at the end of the year, so since Rituxan is considered “off label”, I might not be able to use it again when my ITP comes back. This is why the OPEN Act is so important to me. I want to be able to continue living a normal life.
So Senators, I ask that you support the OPEN Act and work together with representatives in the House to get the 21st Century Cures Initiative on President Obama’s desk by the end of the year. I know it can be done, and patients like me cannot wait any longer. We need more treatments and cures, before it’s too late!